Low domestic production and strict controls on the import and distribution of opioid medicines has created a shortage of pain-killing drugs, forcing some to turn to the burgeoning black market for relief.
By KYAW YE LYNN | FRONTIER
A CALM and peaceful atmosphere prevails at the U Hla Tun Hospice, a palliative care facility in Yangon’s East Dagon Township where treatment is provided free of charge to the neediest of terminally ill cancer patients.
Patients at the hospice, which opened in 2000, have access to pain-relieving medicines, such as tramadol, an opioid medication.
“We provide painkilling drugs regularly to those who are in chronic pain – generally one dose every four hours, but it depends,” says the hospice’s chief nurse, Daw Myint May. “Whenever a patient complains that they are in pain, we give them medicine.”
Myint May said the hospice had relied heavily on former Brigadier-General David Abel, who served as chairman of the U Hla Tun Hospice (Cancer) Foundation from 2006 until his death in January this year, to help secure its supply of pharmaceuticals for palliative care.
Support more independent journalism like this. Sign up to be a Frontier member.
It buys powerful drugs from the state-run Myanmar Pharmaceutical Industry Enterprise, she said. “We have [had] a steady supply of necessary pharmaceutical products from BPI until now,” she said, referring to the enterprise by its former name, Burma Pharmaceutical Industries.
But many other health facilities are unable to legally access the pain-relieving drugs that their patients need.
U Than Sein, the chairman of the People’s Health Foundation, a non-government organisation, said that successive governments had tightly restricted the import, sale and use of pain-relieving drugs because it regarded them as addictive.
“They apply very strict regulations to prevent them from being misused,” he said.
Than Sein said the restrictions on the pain-relieving drugs were too strict and created an imbalance between demand and supply.
Many health facilities had little option but to instead buy the drugs on the black market, where they were more expensive, he said.
The black market
Food and Drug Administration director general U Khin Zaw agreed that the black market was a response to the tight restrictions.
He said only a few pharmaceutical retailers were able to satisfy the licence conditions for storing and dispensing prescription and controlled drugs.
At the same time, Khin Zaw said Myanmar’s “long border and low capacity to prevent” the illicit trade in controlled medicines meant they could easily be smuggled into the country.
“I think store owners are afraid of being punished for failing to follow the rules and so do not apply for a licence,” Khin Zaw said an interview at FDA’s headquarters in Nay Pyi Taw on August 6. “Instead, they sell controlled drugs without having the proper licence and in this way, the black market [for controlled drugs] occurs.”
Khin Zaw said the ministry is planning to ease the restrictions to make it easier to buy the pain-relieving drugs, including by allowing sales through pharmacies at private clinics if they adhere to the licence conditions. Currently, only pharmacies at public and private hospitals are permitted to sell controlled drugs.
The ministry is also planning to sell or provide a month’s supply of the drugs to patients who live far from hospitals or clinics. There are also plans to ease restrictions on prescribing the drugs.
Khin Zaw said the changes were the result of the FDA responding to feedback from the public, MPs and the media, as well as the market situation. “We believe the planned relaxations will make these controlled drugs more accessible,” he said.
The 1992 National Drug Law, which was amended in 2013, provided for the creation of the Food and Drug Board of Authority to regulate and control the manufacture, import, export, storage, distribution and sale of food and drugs. It imposed strict rules and regulations on several classes of pharmaceuticals by issuing a series of notifications.
The FDA, which is under the Ministry of Health and Sports, took over responsibility for granting access to controlled drugs after the law was amended in 2013.
ns-17.jpg
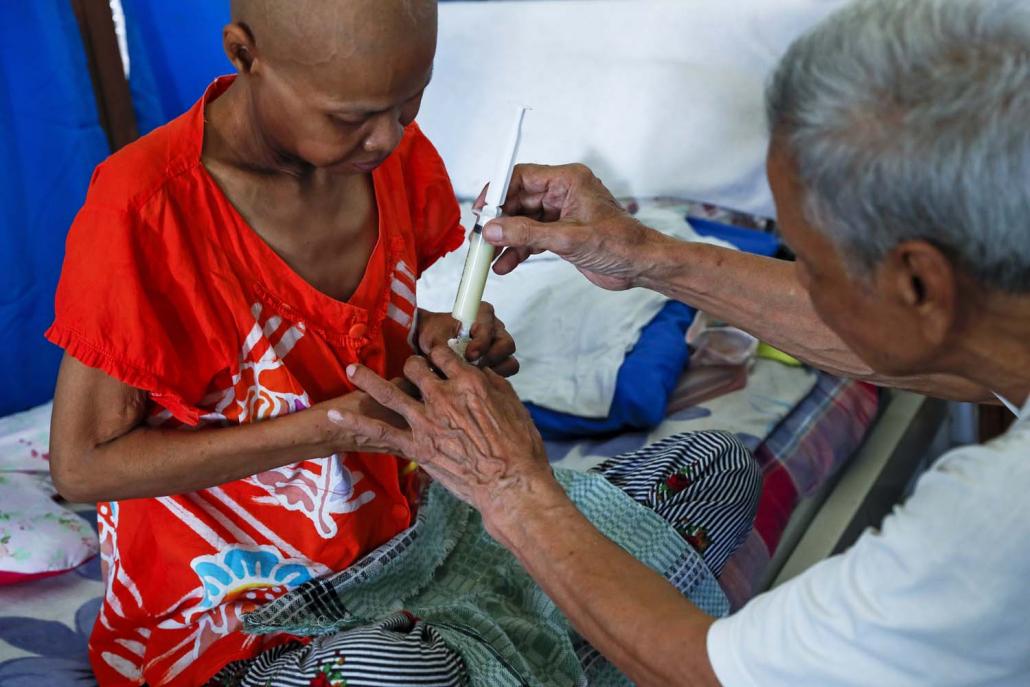
A patient at the U Hla Tun Hospice in Yangon’s East Dagon Township prepares to receive a dose of pain-killing medicine. (Nyein Su Wai Kyaw Soe | Frontier)
Quotas and red tape
Each year, the FDA compiles data on the estimated volumes needed by private hospitals and clinics, while government ministries, including health and defence, do the same for the public sector. The data is provided to the government’s Central Committee for Drug Abuse Control, which submits the list of controlled drugs required for the coming year to the International Narcotics Control Board, a United Nations agency. With the approval of the INCB, the FDA then allows the private sector to import controlled drugs four times a year.
Despite the shortage of medicines, Khin Zaw said Myanmar had never met the yearly import quota approved by the INCB.
“For example, the board approved the importation of 100 kilograms of a highly controlled medicine last year. But only about half of the quota was imported by the end of the year,” he said.
However, quotas for some individual drugs are exceeded, Khin Zaw said. The FDA has allowed the import of 15 kinds of controlled medicines, but private companies mostly apply to import just a few types, such as tramadol and morphine.
FDA records show that last year six private companies had applied to import three products from a list of 10 normal controlled medicines and 11 companies had applied to import two of five highly controlled pharmaceuticals, he said.
“The most imported medicines are only three or four kinds, such as tramadol,” Khin Zaw said. “That’s probably one reason why the country is facing a shortage of controlled medicines.”
Asked why the government did not simply request a higher quota for the handful of medicines that are widely used in Myanmar, Khin Zaw said it was because the INCB would “definitely not allow the amount requested”.
But limited availability is not the only barrier to private health facilities acquiring controlled medicines.
One doctor who runs a private hospital told Frontier earlier this year that the procedure that private hospitals, clinics and pharmacies must follow to apply for a licence to sell controlled medicines over the counter or to patients receiving treatment was complicated and lengthy.
“There are many, many steps of applying, and each step takes up to six months because it needs recommendations from several departmental heads and most steps need approval from a central-level organisation,” the doctor said, referring to CCDAC in Nay Pyi Taw.
Many private clinics and hospitals, including the doctor’s own, are not prepared to wait, and so proceed without a licence. “I have been operating my hospital for one-and-a-half years but my licence is yet to be approved,” he said. “It means my hospital has no licence and faces closure if it was found out by the authorities.”
Those caught providing controlled medicines without a licence could face serious charges under the National Drug Law, the Narcotic Drugs and Psychotropic Substances Law and the Myanmar Medical Council Law.
But the doctor also said he had no choice other than to rely on the black market to buy the controlled medicines that his hospital needed.
“You go to big pharmaceutical stores, but don’t get the necessary medicines. So what do you do? Do operations without any pain-relieving medicines? The conditions force us to buy the necessary controlled drugs from stores that have not applied for a licence to sell over the counter. Sometimes we have to use medicines that the importing companies have not registered for FDA approval,” he said.
The Myanmar Private Hospitals’ Association and the Myanmar Pharmaceuticals and Medical Equipment Entrepreneurs’ Association declined to be interviewed for this story.
Home grown?
There have been suggestions that Myanmar should encourage the domestic production of controlled drugs.
“In many countries, such as neighbouring India, [opium] poppy plantations are permitted for medical purposes,” said Than Sein from the Public Health Foundation. “Our country doesn’t allow it, but is ranked as one of the world’s top opium producing countries. This is strange.”
According to the FDA, the MPIE and a few private companies are producing some controlled medicine products in Myanmar.
“However, the amount is only about one-third of the controlled medicines required for the country,” said Khin Zaw, adding that most of the raw materials were imported.
MPIE managing director U Ko Ko Aung told Frontier in May that most of the controlled medicine produced by MPIE was bought by the health ministry, while the private sector mainly relied on imports.
(Ko Ko Aung has since resigned in the wake of the Anti-Corruption Commission arresting Dr Aung Zaw, the head of the MPIE factory in Yangon’s Insein Township, on corruption charges.)
But in an effort to meet domestic demand, MPIE has already significantly increased production of some controlled medicines and plans to expand output further this financial year, according to production targets Ko Ko Aung provided to Frontier.
In 2018-19 it expects to produce 9 million 50-milligram capsules of tramadol (up from 1.15 million in 2017-18), 500,000 ampoules of tramadol injections (not produced in 2017-18) and 2 million morphine sulfate tablets (up from 877,000 in 2017-18), the document shows.
One challenge, Ko Ko Aung said, was a shortage of raw materials for the manufacture of controlled medicines.
Some of the opium seized in Myanmar was used to make controlled medicines, he said, but opium that had been contaminated by chemicals was unsuitable.
“Unlike in the past, the opium seized today is not pure. Just a small portion of the seized opium is usable for the production of controlled medicine,” he said. “So most of the raw material has to be imported from other countries.”